Non-Invasive Approaches for Treating Depression and Anxiety Without Medication: Benefits for Teens

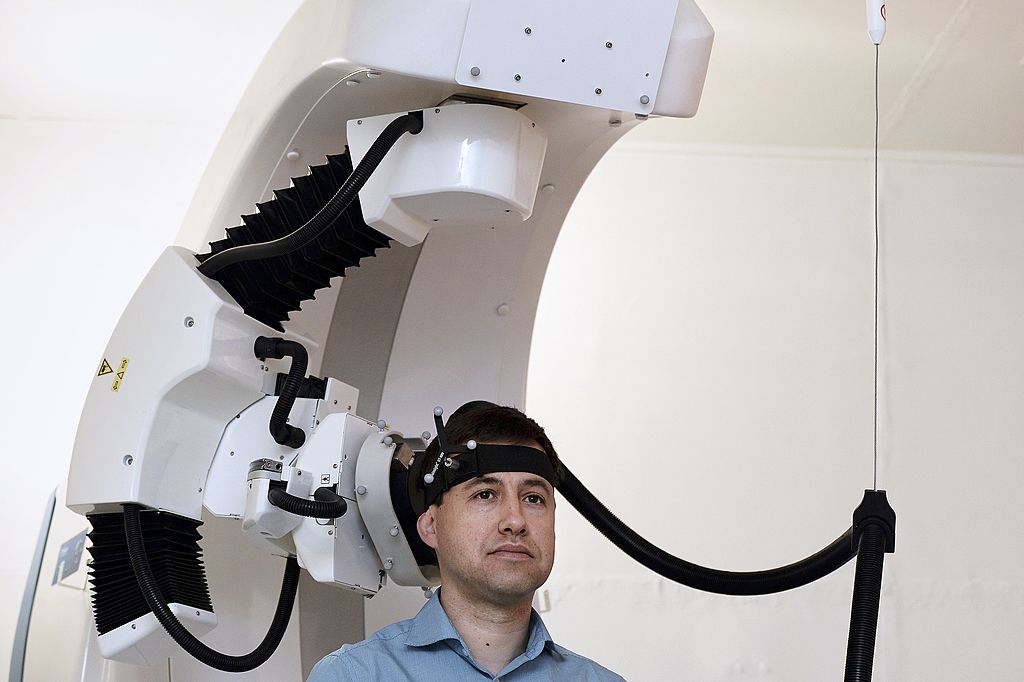
Innovative Treatment for Adolescent Depression: Transcranial Magnetic Stimulation
Adolescence can be a tumultuous period, often characterized by emotional and psychological challenges. Among these, depression and anxiety are increasingly prevalent, impacting teens’ academic performance, interpersonal relationships, and familial connections. While conventional treatment often includes prescriptions for antidepressants, an alternative method gaining traction is Transcranial Magnetic Stimulation (TMS). This non-invasive therapy provides a promising avenue for alleviating the symptoms of depression and improving mental health outcomes, particularly for younger individuals.
Understanding Transcranial Magnetic Stimulation
TMS is a brain stimulation technique that was first introduced in 1985 and garnered approval for clinical use in the United States in 2008. It employs a non-invasive approach to stimulate specific areas of the brain, primarily the dorsolateral prefrontal cortex, which plays a critical role in mood regulation. By sending targeted magnetic pulses to these regions, TMS activates underperforming neural pathways, promoting enhanced emotional well-being.
Typically, patients undergo sessions lasting between 20 to 40 minutes while seated comfortably. Following a series of treatments, many individuals report improved mood and reduced symptoms of depression. TMS is well-tolerated, with minimal side effects, making it an attractive option for adolescents, who may be deterred by the discomfort associated with more invasive procedures.
Advantages of TMS Over Conventional Antidepressants
A significant body of research underscores TMS’s effectiveness as a treatment for depression, particularly in cases where traditional antidepressants have failed. Studies indicate that approximately 50% to 60% of individuals receiving TMS exhibit a “clinically meaningful response,” with one-third experiencing complete remission of symptoms. Unlike pharmaceuticals, which often carry a heightened risk of adverse effects—including increased suicidal thoughts among youths—TMS presents a safer alternative. The FDA notably mandates a “black box” warning for antidepressants due to these potential risks, concerned particularly with younger populations.
Furthermore, the withdrawal symptoms associated with abruptly discontinuing antidepressant medication, such as dizziness and nausea, can be distressing for patients. In stark contrast, TMS does not induce such withdrawal-related complications.
Conclusion
As mental health issues among teenagers become an escalating concern, exploring diverse treatment modalities is imperative. TMS stands out as a non-invasive, effective treatment with promising results. While it does not claim to provide a permanent solution to depression, many patients report sustained improvements long after completing their sessions. By integrating TMS into therapeutic options for adolescent mental health, healthcare providers can offer a pathway toward recovery that prioritizes both efficacy and safety—an essential balance in treating sensitive populations.
As research continues to evolve, TMS may very well redefine the approach toward managing depression in adolescents, guiding them through their formative years with improved well-being and resilience.






