Parents report that infants who consumed ByHeart formula fell ill prior to botulism outbreak.

Investigation into Infant Botulism Cases Sparks Parental Concerns Over ByHeart Baby Formula
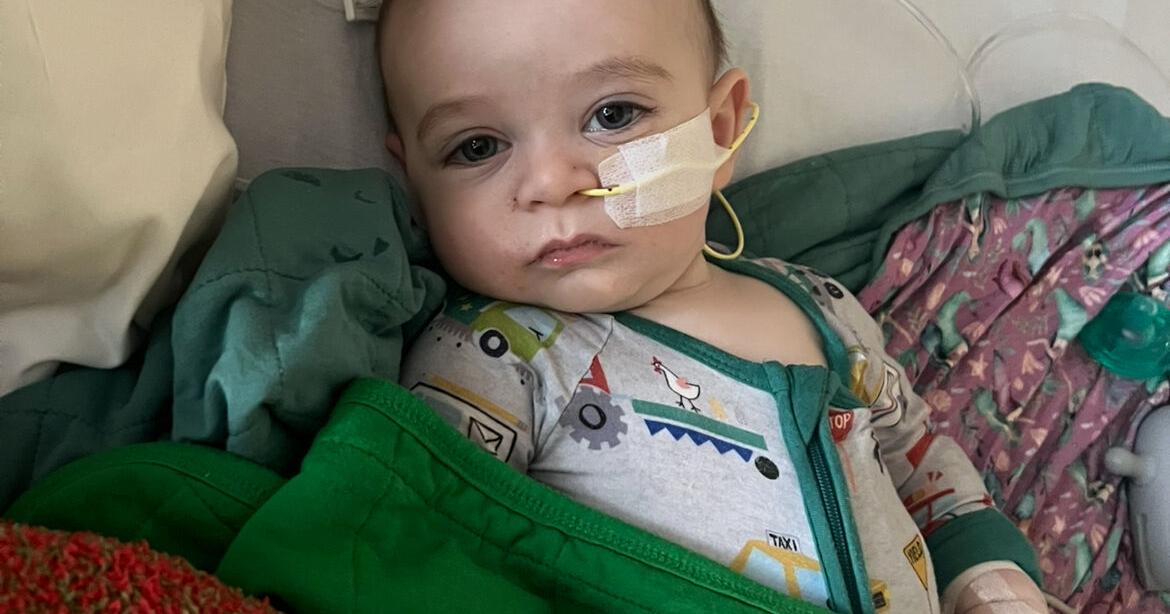
In recent developments, public health officials are grappling with more than 30 reported cases of infant botulism linked to ByHeart baby formula, a situation that has drawn significant concern from parents across the nation. These alarming incidents have been under investigation since August, raising further questions about the safety protocols surrounding baby food products.
California health authorities confirmed that six infants in the state were treated for botulism after consuming the ByHeart formula, with these cases occurring between November 2024 and June 2025. This timeline is notably concerning, as these cases preceded the current outbreak by several months. The overlap in timelines has prompted an inquiry into whether there may have been early warning signs ignored by both the manufacturers and regulatory agencies.
Botulism, a rare but potentially life-threatening illness caused by toxins produced by the bacterium Clostridium botulinum, poses serious risks to infants, whose immune systems are still developing. The symptoms can include paralysis, difficulty in breathing, and gastrointestinal distress, which can escalate rapidly without prompt medical attention. Given the vulnerability of the infant population, these cases highlight the critical need for stringent safety measures and vigilant monitoring within the baby food industry.
Parents are now seeking clarity on the situation, voicing their concerns about the connection between previous illnesses and the current outbreak. Many have expressed frustration, feeling that their children’s health concerns were overlooked until the situation reached a more urgent level. This sentiment underscores the necessity for increased transparency from companies like ByHeart and regulatory bodies regarding product safety and public health alerts.
In response to these incidents, health officials are urging any parents who suspect their children may have been affected to seek medical attention immediately. Awareness campaigns are also being ramped up to educate parents about the signs of botulism and the importance of reporting any adverse reactions to infant formulas.
As investigations continue, the outcome may have implications for regulatory practices within the food manufacturing sector, particularly concerning infant nutrition products. Parents are left to navigate the challenges of ensuring their infants’ safety while waiting for answers that could prevent further incidents. As the situation develops, the focus remains on safeguarding infant health and restoring trust in baby formula products.
The current crisis reinforces the importance of collaboration among manufacturers, health officials, and parents alike to enhance monitoring systems, public health communication, and ultimately, the safety of vulnerable populations.



